PROTECT YOUR DNA WITH QUANTUM TECHNOLOGY
Orgo-Life the new way to the future Advertising by Adpathway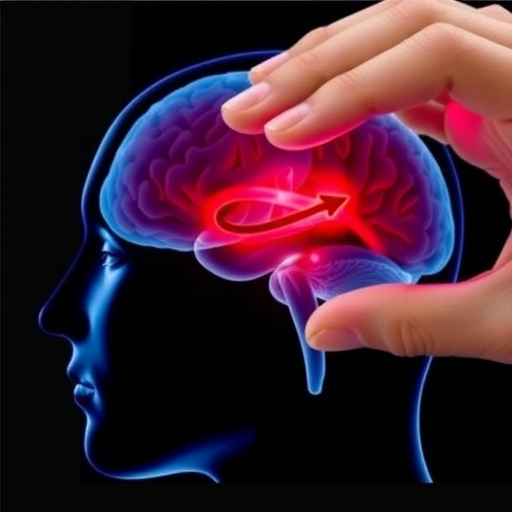
In a groundbreaking advancement poised to revolutionize neurological medicine, researchers across North America have detailed the first comprehensive technical methodology utilizing focused ultrasound to safely and reliably disrupt the blood-brain barrier (BBB). This pivotal research, recently published in the journal Device, stems from a collaborative effort led by Dr. Graeme Woodworth of the University of Maryland School of Medicine (UMSOM), alongside colleagues at Brigham and Women’s Hospital in Boston and other premier institutions. Their work paves the path for expanding the use of focused ultrasound technology as a transformative tool for enhancing the precision and effectiveness of treatments for brain tumors and various neurological conditions.
The blood-brain barrier represents one of the foremost challenges in neurotherapeutics and brain disease management. This complex, selectively permeable membrane shields the brain’s delicate microenvironment from harmful agents such as toxins and pathogens, but at the cost of limiting access to potentially life-saving medications. Consequently, delivering chemotherapy agents or novel therapeutics to brain tissue in adequate concentrations has long been stymied by the BBB’s formidable protective function. Focused ultrasound, in conjunction with microbubble contrast agents, offers a non-invasive avenue to transiently and locally open this barrier, facilitating the controlled passage of drugs without compromising overall cerebral protection.
To rigorously characterize how focused ultrasound can enable this process with precision and reproducibility, Dr. Woodworth and his team conducted an extensive study involving 34 glioblastoma patients. These participants underwent up to six monthly cycles of treatment, culminating in an impressive dataset of 972 individual sonications—targeted ultrasound pulses aimed at specific brain regions. This large-scale effort allowed the team to meticulously analyze how different ultrasound parameters correlate with successful BBB disruption. Vital to this endeavor was the use of acoustic emissions monitoring: the capture of sound waves emitted by microbubbles oscillating in response to the ultrasound field, which serves as a real-time biomarker correlating with the degree of BBB opening.
Acoustic emissions, generated as microbubbles respond to the ultrasound energy, provide a novel, quantitative feedback mechanism enabling clinicians to fine-tune the treatment dose and target. As Dr. Woodworth explains, these signals allow for reliable prediction of BBB opening events, fostering safer and more effective therapeutic delivery. By correlating the acoustic signatures with MRI imaging and clinical outcomes, the team developed dosing guidelines that transcend individual device differences and patient variability, establishing a unifying framework for blood-brain barrier modulation through focused ultrasound across diverse clinical environments.
The study’s technical rigor is complemented by its translational importance. Previously, the lack of standardized protocols and monitoring impeded broader clinical adoption of ultrasound-mediated BBB opening. This research, therefore, marks an essential milestone, elucidating the spatial control and dosing strategies necessary for consistent, reproducible BBB disruption. Consequently, this advancement promises to accelerate the integration of ultrasound-facilitated drug delivery into routine neuro-oncological care, ultimately enhancing therapeutic efficacy for glioblastoma and potentially other neurological conditions.
The procedural basis for this treatment uses microbubbles—microscopic, inert gas-filled spheres introduced intravenously—which underlie the focused ultrasound technique. When exposed to low-intensity ultrasound waves, these microbubbles oscillate rhythmically within the cerebral vasculature. Their mechanical activity induces transient, microscopic disruptions in the tight junctions of the endothelial cells that compose the BBB, creating temporary pores through which therapeutic agents can pass. Crucially, this process is reversible and highly localized, minimizing off-target effects and preserving overall brain function while improving drug penetration.
Dr. Pavlos Anastasiadis, Assistant Professor of Neurosurgery at UMSOM and a co-author on the study, highlights the mechanistic underpinnings of this phenomenon. The oscillation of microbubbles within the ultrasound energy field leads to subtle mechanical perturbations of the blood vessel walls in the brain, enabling a safe and reversible opening of the BBB. These events can be monitored in real time using advanced imaging and acoustic emission technologies, allowing clinicians to control the extent and location of barrier disruption with unprecedented precision.
The lineage of this work extends back to seminal experiments in the early 1990s at Brigham and Women’s Hospital’s Focused Ultrasound Lab, where microbubbles were first explored as agents for BBB modulation. Building on these foundational discoveries, senior author Dr. Alexandra J. Golby, Director of Image-Guided Neurosurgery at Brigham and Women’s Hospital, emphasizes that the present study validates a clinically feasible approach to repeatedly open the BBB in glioblastoma patients ahead of chemotherapy cycles. This iterative opening holds promise to vastly improve therapeutic accumulation within tumors, potentially enhancing survival and quality of life.
Data from this investigation were derived from a subset of patients enrolled in ongoing clinical trials spearheaded by Dr. Woodworth. These trials are critically assessing the clinical impact of ultrasound-facilitated BBB opening for enhancing the delivery of standard-of-care chemotherapy in glioblastoma. The research team plans to publish detailed clinical outcomes from these broader trials imminently, promising further valuable insights into safety, efficacy, and patient benefit.
Dr. Taofeek K. Owonikoko, Executive Director of the University of Maryland Marlene and Stewart Greenebaum Comprehensive Cancer Center, noted the far-reaching implications of these findings for the field of neuro-oncology and beyond. The study’s data offer the first detailed technical description of acoustic emissions dosing, a cornerstone for clinical and regulatory progress in the adoption of focused ultrasound as a precision treatment modality. This work solidifies the foundation on which larger pivotal trials and multi-center studies can build.
One such major trial underway is LIBERATE (NCT05383872), a diagnostics-focused study in glioblastoma patients. Co-led by Dr. Woodworth, this trial leverages MRI-guided focused ultrasound to assess not only therapeutic delivery but also diagnostic enhancement capabilities, representing a frontier in personalized medicine for brain cancer. The consortium ReFOCUSED—encompassing over 20 research sites across North America—collaborates on these efforts, aiming to harness focused ultrasound technology to transform clinical outcomes in brain disease through improved drug delivery and imaging.
This research was generously supported by Insightec Inc., the manufacturer of the focused ultrasound devices utilized, along with funding from the Focused Ultrasound Foundation. Their combined support underscores the growing momentum behind ultrasound-enabled therapies, fostering innovation at the intersection of technology, engineering, and clinical neuroscience.
In summary, this detailed elucidation of acoustic emissions-guided dosing and spatial control of BBB opening ushers in a new era in neurotherapeutics. Through meticulous technical exploration, this research offers a blueprint for safely breaching the brain’s protective barrier on demand, thereby expanding the armamentarium against formidable brain cancers such as glioblastoma. As standardized protocols permeate clinical practice, focused ultrasound’s promise as a non-invasive, targeted, and controllable delivery mechanism nears clinical reality, unlocking potential not just in oncology but across the landscape of neurological diseases.
Subject of Research: Focused ultrasound-mediated blood-brain barrier opening for enhanced drug delivery in glioblastoma
Article Title: Acoustic emissions dose and spatial control of blood-brain barrier opening with focused ultrasound
News Publication Date: 25-Aug-2025
Web References:
University of Maryland School of Medicine
University of Maryland Marlene and Stewart Greenebaum Comprehensive Cancer Center
Clinical Trial LIBERATE (NCT05383872)
Journal Device DOI
Image Credits: University of Maryland School of Medicine
Keywords: Blood brain barrier, Glioblastomas, Cancer
Tags: blood-brain barrier disruptionbrain tumor treatment innovationschallenges in brain disease managementcollaborative research in neurosciencedrug delivery to brain tissueenhancing chemotherapy efficacyfocused ultrasound technologymicrobubble contrast agentsneurological medicine advancementsnon-invasive neurotherapeuticsrevolutionizing brain health treatmentssafe medical imaging techniques


 2 hours ago
8
2 hours ago
8

















 English (US) ·
English (US) ·  French (CA) ·
French (CA) ·